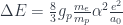If everything I knew about Arecibo, I learned from watching the James Bond flick, Golden Eye, I would be sorely disappointed when I arrived in Cuba and couldn’t find the telescope. (I am not sure how I got to Cuba, seeing as I am a U.S. citizen; perhaps I joined a dance troupe that was allowed a visit in order to learn an authentic rumba.) The world’s largest, single-dish radio telescope is NOT in Cuba, but in the Puerto Rican jungle, just south of the town of Arecibo. The location was chosen for two reasons: 1) if you are going to put a 305-m dish in the ground, you might as well put it in a place where there’s a hole! The karst terrain in Puerto Rico is full of natural sink holes. 2) The observatory was constructed near the equator (where all of the planets in our solar system are visible) so that the radar used to study the ionosphere could also be used for planetary observations.
If everything I knew about Arecibo, I learned from Golden Eye, I would expect a sleek, state-of-the-art, enormous control room. In reality, the control room is pretty state-of-the-art – no complaints there – but I would not exactly call it sleek. There is a bookshelf full of supposed entertainment for observers that I have NEVER seen anyone touch, like CDs of Bette Midler and The Best of Herbie Hancock, or a board game called India with no instructions. There are also the sounds! There are speakers that broadcast the audio happenings inside the dome and at the tie-downs (the cables that keep the platform steady), and occasionally a stray coqui will get trapped and sing for the observers all night. Also, if you abort an observation, you hear the loud sound of a toilet flushing. Speaking of flushing toilets ….
If everything I knew about Arecibo, I learned from Golden Eye, I would think the dish filled with water when it was not in use. Not only is this totally untrue, it’s impossible! The dish is not actually solid, but is made up of almost 40,000 aluminum panels (each 3 feet by 6 feet) that are all individually adjusted to maintain the spherical curvature of the dish. Here is a photo taken from underneath to give you an idea of the openness of the panels:

To find out more “real” facts about the observatory that don’t come from a movie starring Pierce Brosnan, watch Contact instead! No, just kidding. Check out the observatory’s official homepage.
The ALFALFA Survey is trying to detect galaxies through emission at the ’21cm line’. In fact, you’ll see mention of the 21cm line throughout extragalactic radio astronomy as it is one the most common observations. This might lead you to wonder: What is the 21cm line? And why do we want to observe it? For now, I’ll address the first of those questions; you’ll learn all about why the 21cm line is interesting in a later post.
Alternately, this post could be titled: “A Quick and Dirty Introduction to the Hydrogen Atom”. The ’21cm line’ is a specific transition in the hydrogen atom, so called because the energy of the transition corresponds to a photon with a wavelength of 21 cm. Physicists love hydrogen because it’s the simplest atom – one proton and one electron – meaning it can be solved exactly. Astronomers love hydrogen because it fills the universe and observing it in different states offers lots of information about conditions found in astronomical situations.
Most people are familiar with the simple Bohr model of the atom where electrons orbit the nucleus in quantized orbits. Of course, like many physics explanations, the Bohr model isn’t accurate but it does provide a convenient picture for much of the physics so we continue to use it. The transitions in atoms that most people are familiar with are electronic transitions. The orbits of electrons in an atom have quantized energy states and there is a specific energy associated with a move from one orbit to another. For hydrogen, these electronic transitions have energies that release photons in the ultraviolet, optical and infrared parts of spectra. The energies associated with the 21 cm line are much, much lower. In order to understand where the 21cm line comes from we have to look further at the structure of hydrogen. The first set of corrections made are termed “fine structure” and account for the fact that the base calculation ignores relativistic effects. These aren’t the corrections that cause the 21 cm line, though. For that we need to go to a second set of corrections (which have an even smaller change in energy levels) – the “hyperfine structure” of hydrogen.

An illustration of the Bohr model of the atom
The hyperfine structure refers to the weak interaction between the spins of the proton and electron. Spin is an extremely quantum idea and hard to describe with a classical analogy. The best way to think of it is as particles spinning on their own axis – it behaves as an angular momentum term in many ways. However, it does have some odd properties, which we will ignore for now. If you keep the simple picture of a charged particle spinning in mind, you can imagine a slight magnetic field resulting as moving charges cause magnetic fields. Similarly, the proton creates a slight magnetic field. The interaction of these two magnetic fields results in the 21 cm transition.
The electron and proton can both be thought of as magnetic dipoles generating magnetic fields. There is then an energy difference between when the dipole moments of the electron and proton are aligned or anti-aligned. The state where the two spins (or magnetic moments) are anti-aligned is a lower energy state; when the atom transitions from a “spin-up” electron to a “spin-down” electron, a photon with a wavelength of 21 cm (corresponding to the energy difference between the levels) is released.

Illustration of the 21 cm transition
I’m going to try ending posts with the math so that it’s available if you’re interested and easy to skip if you’re not – hopefully everything I write will be clear without any equations.
The energy of a photon is related to its frequency by 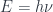 where
where  is the frequency and
is the frequency and  is Planck’s constant.
is Planck’s constant.
If you would like to know the wavelength of this photon, you can find it from the speed of light: 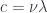 where
where 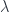 is the wavelength.
is the wavelength.
If you would like to calculate the energy of the hyperfine transition a back-of-the-envelope approximation is:
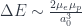
where  is the Bohr radius of an atom and
is the Bohr radius of an atom and 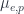 are the magnetic moments of the electron and proton, respectively.
are the magnetic moments of the electron and proton, respectively.
Substituting in for the magnetic moments this becomes,

where  is the degeneracy of the proton ( ~2.79),
is the degeneracy of the proton ( ~2.79),  are the masses of the electron and proton, respectively,
are the masses of the electron and proton, respectively, 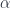 is the fine structure constant (~1/137), and
is the fine structure constant (~1/137), and  is the charge of the electron.
is the charge of the electron.
This approximation is very close to the true calculation using quantum mechanics: